MelodyValve.org
Enter a key term, phrase, name or location to get a selection of only relevant news from all RSS channels.
Enter a domain's or RSS channel's URL to read their news in a convenient way and get a complete analytics on this RSS feed.
Unfortunately MelodyValve.org has no news yet.
But you may check out related channels listed below.
[...] conjunction with the doctors to make the necessary modifications to the Melody valve for the mitral position, and pursue expedited approval with the FDA for an indicated use. To these ends, we ask the [...]
[...] , to move past trailblazer and Boston-innovation-centric use of the Melody, and make the Melody valve an accepted and FDA approved treatment for children with mitral valve disease. We understand [...]
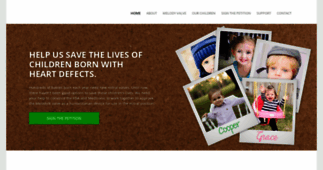
[...] Pwky NE LC -110 Minneapolis, MN 55432 Dear Omar, We are parents of children who, as mitral valve disease threatened their lives, received Melody valves in their mitral positions. If it were just [...]
[...] valve, but that they didn’t know how long she would make it given her severely dysfunctional mitral valve. Not content with simply watching as their daughter’s health deteriorated, [...]
[...] many more. Milana is the daughter of Janelle and Nick Foligno. Milana was born with severe mitral valve disease. At only days old, Janelle and Nick were told that she needed to grow quite a bit [...]
[...] Therapies Medtronic Sitaram Emani, MD Boston Children’s Hospital Department of Cardiac Surgery Audrey Marshall, MD Boston Children’s Hospital Department of Cardiology Wayne Tworetzky, MD [...]
[...] of decreasing IDE cycle and approval times, increasing the number of early feasibility/first-in-human IDE studies, and leaning towards post-market data collection when the benefits of [...]
[...] the letter below, and feel free to comment. March 28, 2014 Omar Ishrak Chairman and Chief Executive Officer Medtronic 710 Medtronic Pwky NE LC -110 Minneapolis, MN 55432 Dear Omar, We are parents of [...]
[...] the number of early feasibility/first-in-human IDE studies, and leaning towards post-market data collection when the benefits of earlier access to devices would benefit the patients. [...]
[...] of Food and Drug US Food and Drug Administration Bram D. Zuckerman, MD Director of Cardiovascular Devices US Food and Drug Administration Jeffery E. Shuren, M.D., J.D. Director, Center for Devices [...]
[...] conjunction with the doctors to make the necessary modifications to the Melody valve for the mitral position, and pursue expedited approval with the FDA for an indicated use. To these ends, we ask the [...]
Related channels
-
LinuxQuestions.org
LinuxQuestions.org offers a free Linux forum where Linux newbies can ask questions and Linux experts can offer advice. T...
-
GSMArena.com - Latest articles
GSMArena.com is the ultimate resource for GSM handset information. This feed contains the latest articles (news and revi...
-
VOIP-info.org Wiki Changes
RSS feed for changes to www.voip-info.org wiki pages
-
AddThis Blog
News and updates from AddThis
-
Feed Discontinued
KDE-Look.org: Applications for your KDE-Desktop